Family planning is not easy. Challenges include service delivery, negative attitudes towards contraceptives, side effects and timeline adherence. Add limited resources and limited access to clinics and you have the current challenges facing many women in low- and middle-income countries (LMICs). Injectable contraceptives like Depo-Provera, or intramuscular-injections (hereafter Depo), are very popular in sub-Saharan Africa, in part because they are reversible, discrete and long-lasting. With one shot, Depo protects a woman for three months. The downside, as many women know, is it requires a provider to administer the injection, meaning clients must visit a provider every 12 to 14 weeks (quarterly) to receive a re-injection. This isn’t very discrete and is a physical and economic barrier for many women in LMICs. Result: injectable contraceptives have high discontinuation rates.
A retrospective Demographic and Health Survey (DHS) of 21 countries in 2015 found that 41% of women in Malawi discontinued provider-administered injectables within 12-months compared to 32% overall. In the Malawi DHS 2015–2016, 30% of married women who practiced modern family planning used injectables. A recent study showed only half of new users received their first re-injection within the preferred 13-week timeframe (Dasgupta, 2015)! This leaves Ministries of Health, donors, policy planners and other family planning organizations balancing popularity with effectiveness. In many cases discontinuation leads to unwanted pregnancy, so how do countries raise the continuation rates?
Study methods
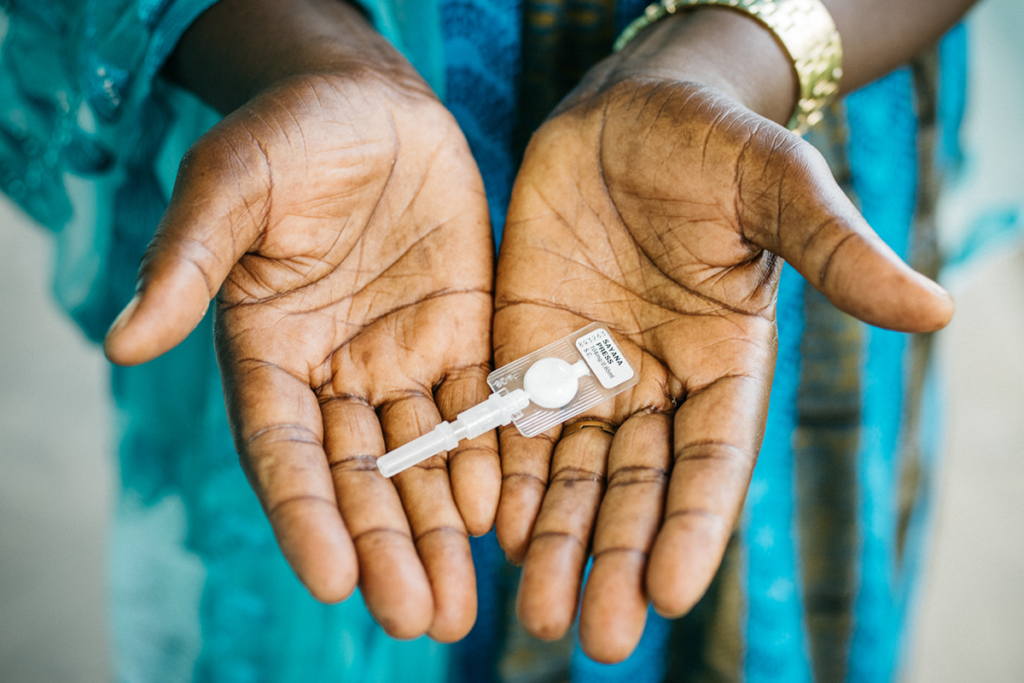
The study uses a formulation of DMPA delivered subcutaneously (in the fat, not the muscle) called DMPA-SC. The formulation is delivered via an injectable technology called Sayana Press. Sayana Press is a small, pre-filled plastic container that is easy to hide. See the picture above. Just like Depo, DMPA-SC is a three-month, progestin-only injectable contraceptive drug and requires a 12 to 14-week re-injection window. Unlike Depo, DMPA-SC delivered in the easy-to-use self-injectable format of Sayana Press has the potential to get rid of quarterly provider visits.
Burke, et al., designed an open-label, randomized control trial (RCT) to compare continuation rates between women who self-administer DMPA-SC and women who receive DMPA-SC from a provider – physicians or community health workers (CHWs) – affiliated with six public-sector clinics in rural Malawi over 12-months. The study ran from September 17, 2015, through February 21, 2017.
The team randomly assigned the women to two groups. The authors needed 367 women in each group (n = 734) to provide 80% power “to detect a 50% increased risk of discontinuation between groups.” The study is strict with the definition of continuation. Women, in either group, had to re-inject every 12 to 14 weeks to continue in the study. If a woman missed one window, the researchers classified her as discontinued.
Injection procedures
Women in the provider-administered group received their injection from a provider at the clinic at the time of enrollment and then told to return to the clinic in 12 to 14 weeks.
For the other group, qualified providers taught the women how to self-inject. User error did not factor in because the study required each woman to successfully inject in front of a provider before continuing. If successful (one woman was not), each woman received three doses of DMPA-SC with written injection instructions. If the woman wanted, she could ask the provider to train another person with her.
Every woman, in both groups, received a note with re-injection dates or clinic visits and a calendar. Beyond that, no woman received any reminders.
Every 15 or 16 weeks, data collectors conducted in-persons interviews with each woman still in the study. Initiating an interview after 14 weeks meant that interviews could not serve as injection reminders. Staff asked for a pregnancy test in the final interview of every woman, even if she received fewer than four injections. For perhaps obvious reasons, pregnancy tests are a common way to judge results in contraceptive studies.
For assurance, the authors blind-reviewed all the records at the end of the study. The study used several statistical tests (Kaplan-Meier methods, Greenwood’s formula, and Fisher’s exact test) to compare groups and all at a significance level of 0.05 for two-sided comparisons.
Results
In the end, the final study data included 731 women. The authors classified 99 women in the self-administered and 199 women in the provider-administer groups as discontinued. For those interested, this discontinuation yielded an incident rate of 8.2 per 100 injection cycles (range: 6.7-10) for the self-administered group, and 20.6 per 100 (range: 17.9-23.7) for the provider-administered group.
According to the study, 178 women discontinued because they missed a re-injection window. However, 47% (83) of those women wanted to continue and only 13% (23) wanted to stop. For a variety of reasons, the desires of the remaining women (40%; 72) are unknown. The authors noted that the demographics, the distribution of reasons for discontinuation, and reports of side effects did not differ significantly across groups. For example, 20% of both groups reported that their husband or partner did not know the woman was seeking family planning services. In my view, this adds weight to the argument LMICs need a discrete, easy-to-use contraceptive.
To strengthen the study, Burke, et al., completed a sensitivity analysis to test a more lenient definition of continuation. This definition relaxes the continuation rate to include any women who reported re-injection even if it was outside the 12 to 14-week window.
With this definition, even more women from the self-administered group continued self-injecting contraceptives. The self-administered group saw an 84% continuation rate while the provider-administered group saw a 53% continuation rate (p < 0.0001).
Discussion
Burke, et al., recognized certain caveats to the study. Specifically, the authors noted that the discontinuation rate for the provider-administered group is higher than in many reported studies but attribute it to their more conservative estimate of continuation rates. In spite of the strong results, I appreciate that Burke, et al., are clear that self-injectable technology using DMPA-SC is not a panacea to the problem of high discontinuation rates. Under the stricter continuation definition over 25% of women still struggled to re-inject during the timeframe.
So, did method matter? In Malawi, the evidence said yes. A woman is more likely to continue using injectable contraceptives (and avoid unwanted pregnancies) if she is able to use new self-injectable technologies.
Photo caption: A woman in Senegal holds subcutaneous DMPA (DMPA-SC or brand name Sayana® Press) in both hands. Subcutaneous DMPA is a lower-dose, all-in-one injectable contraceptive that is administered every three months under the skin into the fat rather than into the muscle.
Photo credit: © 2016 PATH/Gabe Bienczycki, Courtesy of Photoshare